The specific heat capacity of a material is the amount of heat energy required to raise the temperature of one gram of that material by one degree Celsius. It is a measure of how much heat a material can absorb or release without undergoing a change in temperature.

The specific heat capacity of graphite is 0.71 J/g°C, while the specific heat capacity of polyimide is 1.24 J/g°C. This means that it takes more heat energy to raise the temperature of one gram of graphite by one degree Celsius than it does to raise the temperature of one gram of polyimide by one degree Celsius.
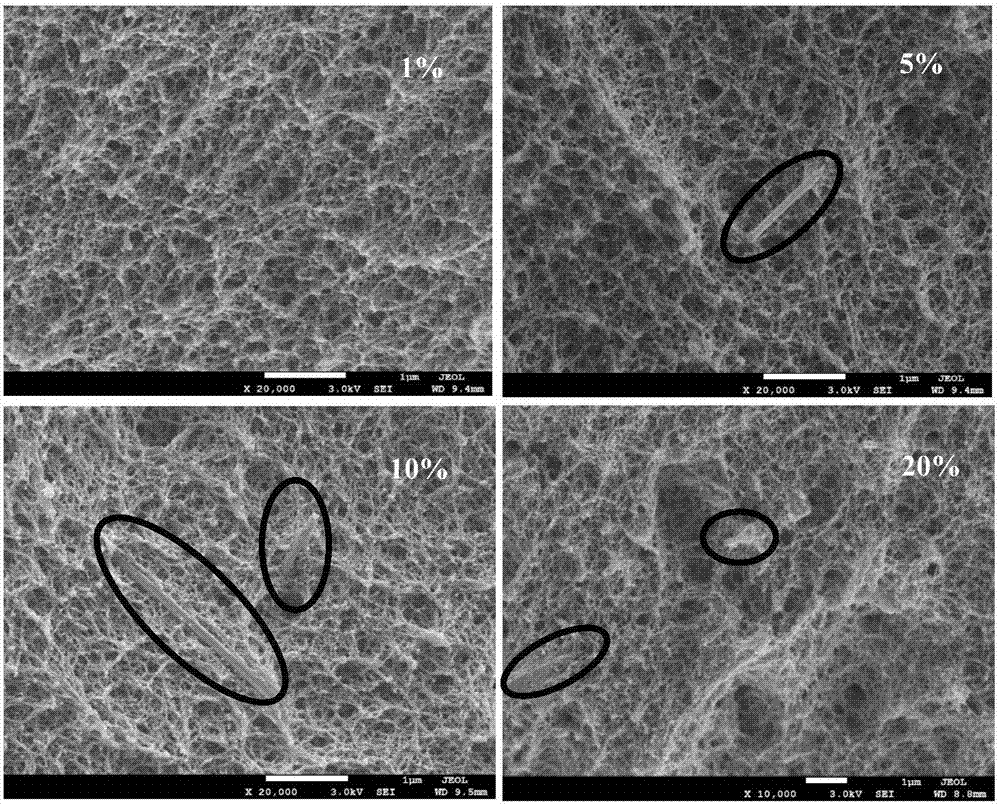
In other words, graphite has a lower specific heat capacity than polyimide. This means that graphite can absorb or release more heat energy without undergoing a change in temperature than polyimide can.
This difference in specific heat capacity is due to the different structures of the two materials. Graphite is a crystalline material, while polyimide is an amorphous material. Crystalline materials have a more regular and ordered structure than amorphous materials, which makes them more dense and allows them to store more heat energy.
The specific heat capacity of a material is an important property to consider when designing devices that generate or absorb heat. Materials with a high specific heat capacity can absorb or release large amounts of heat without undergoing a large change in temperature, making them useful for applications such as heat sinks and thermal batteries.