Polyimide (PI) is a high-performance polymer known for its exceptional thermal stability, mechanical strength, and electrical properties. However, pristine PI is generally insoluble in common organic solvents due to its rigid molecular structure and strong interchain interactions. To overcome this limitation, many strategies have been developed, including the incorporation of flexible segments and the use of polar aprotic solvents.

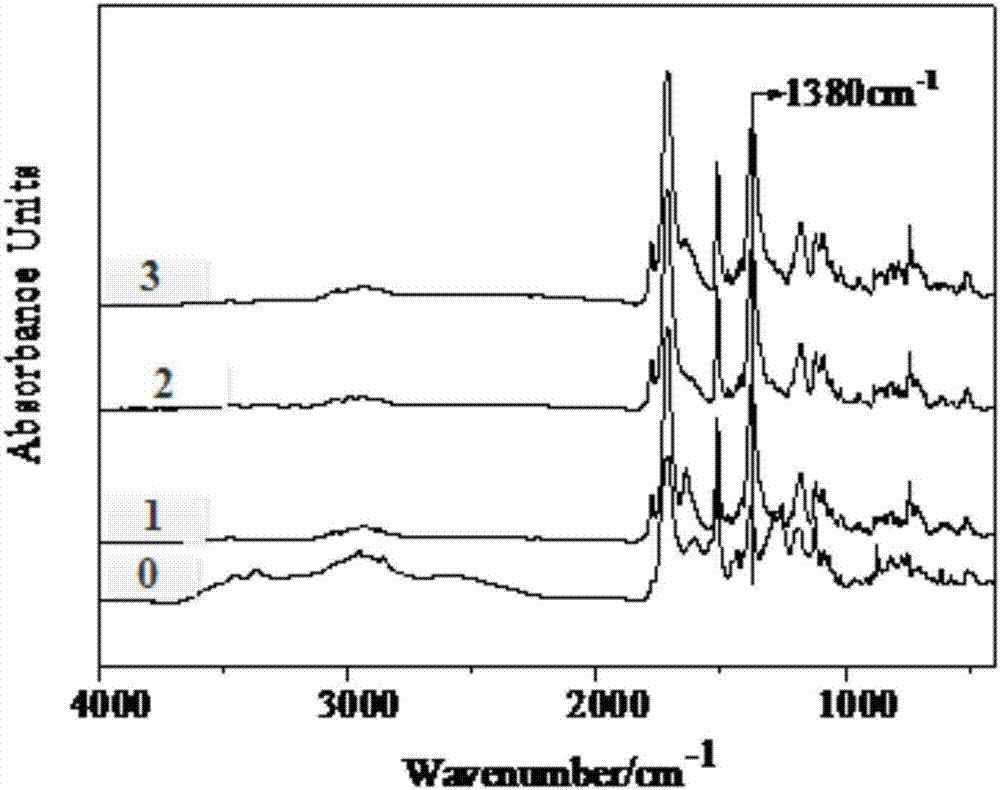
Solubility of Polyimides in DMF
Polar aprotic solvents, such as N,N-dimethylformamide (DMF), are capable of dissolving PI due to their ability to disrupt the interchain interactions and solvate the polymer chains. The specific structural feature in PI that enables its solubility in DMF is the presence of polar imide groups (-C=O-N-) along the polymer backbone. These imide groups can form strong dipole-dipole interactions with the polar aprotic solvent molecules, leading to the partial solvation of the polymer chains.

Role of Crosslinking in Insolubility
Crosslinking is a chemical process that involves the formation of covalent bonds between polymer chains, resulting in a more rigid and interconnected network structure. When PI is crosslinked, the interchain interactions become significantly stronger, and the polymer chains become more entangled, effectively preventing the penetration of solvent molecules into the polymer matrix. As a result, the crosslinked PI becomes insoluble in all common solvents, including DMF.
The insolubility of crosslinked PI is a crucial characteristic that contributes to its exceptional thermal and mechanical stability. The strong bonding between polymer chains restricts chain movement and inhibits the diffusion of gases and liquids, making crosslinked PI an excellent material for high-performance applications, such as microelectronics and aerospace composites.
In summary, the solubility of polyimide in DMF is facilitated by the presence of polar imide groups along the polymer backbone, which can form dipole-dipole interactions with the polar aprotic solvent molecules. Upon crosslinking, the formation of covalent bonds between polymer chains leads to a more rigid network structure, preventing the penetration of solvent molecules and resulting in the insolubility of crosslinked PI.